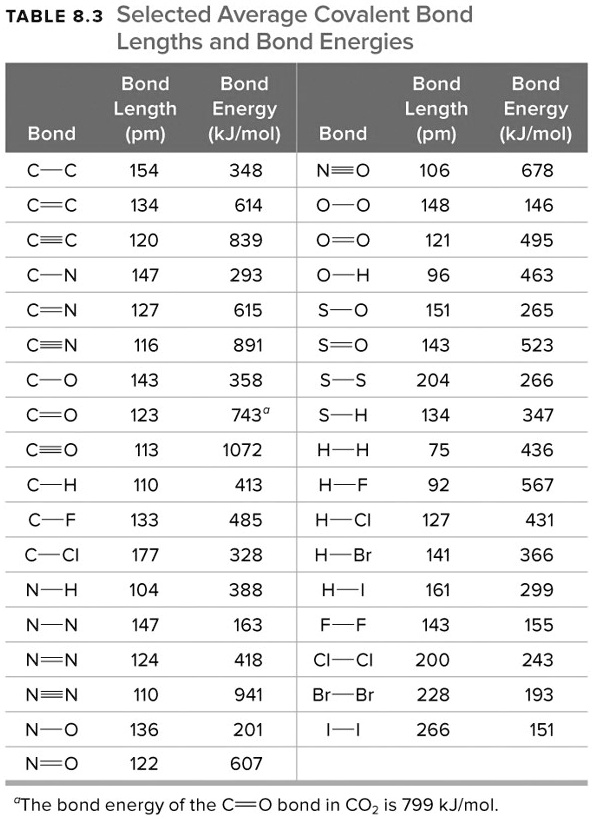
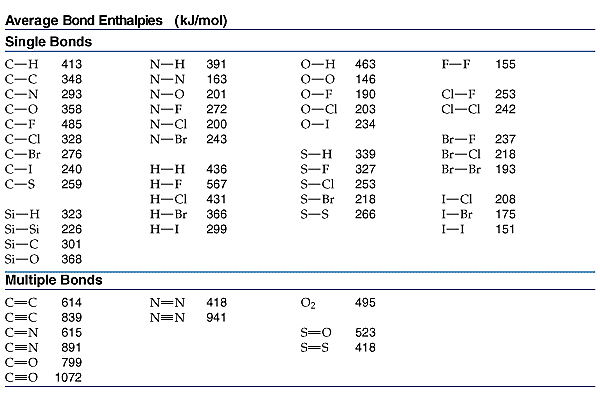
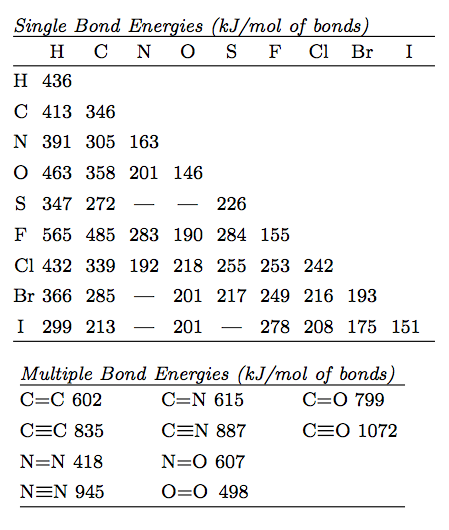
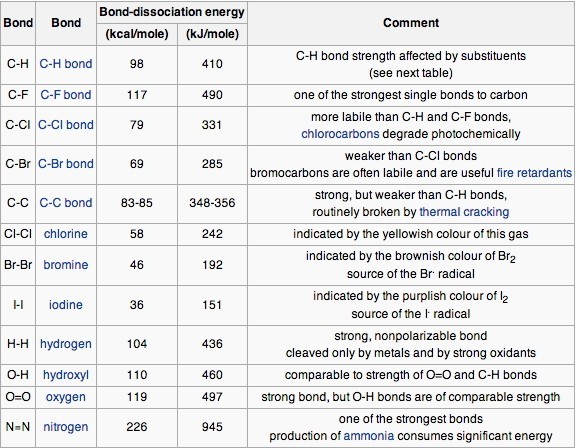
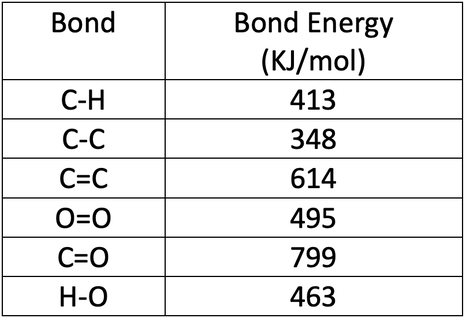
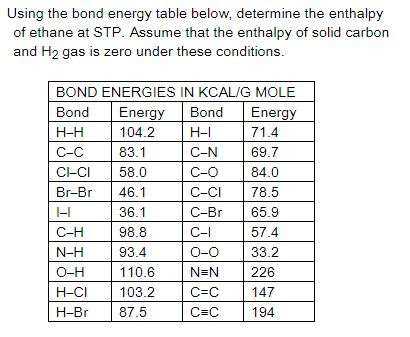
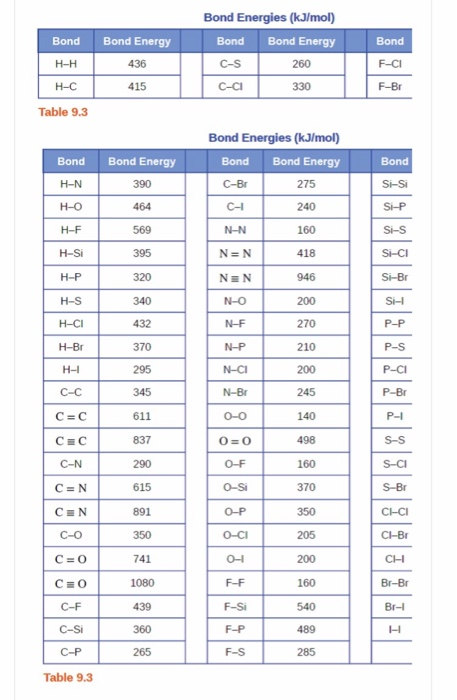
Table 1 Average Bond Energies (kJ/mol) Bond Bond Energy 432 Energy 154 H-H F-F H-F 565 0-H 467 C-H 413 Cl-C1 239 358 614 C-O C=0 1072 745 C=0 C=O (for CO2(g))

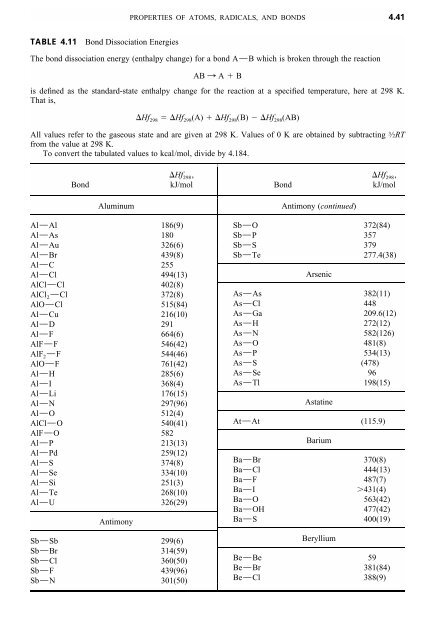
physical chemistry - The source for the N-N bond dissociation energy of 240 kJ/mol in the table - Chemistry Stack Exchange
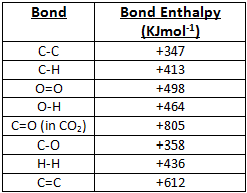
Using the bond energies in Table 7.2, determine the approximate enthalpy change for each of the following reactions: (a) H 2 ( g ) + Br 2 ( g ) → 2
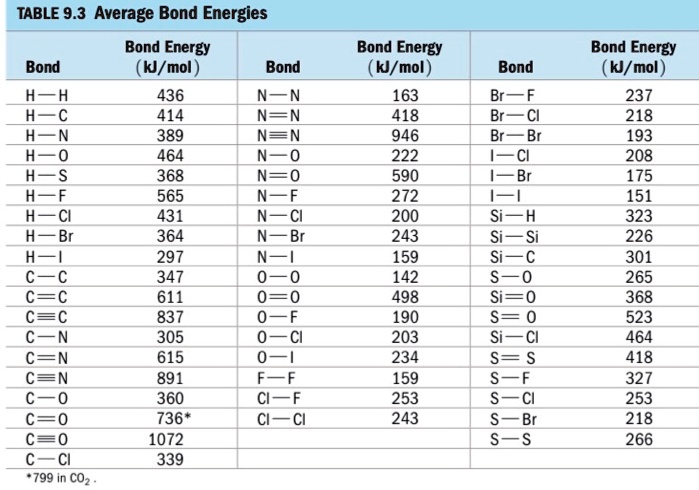
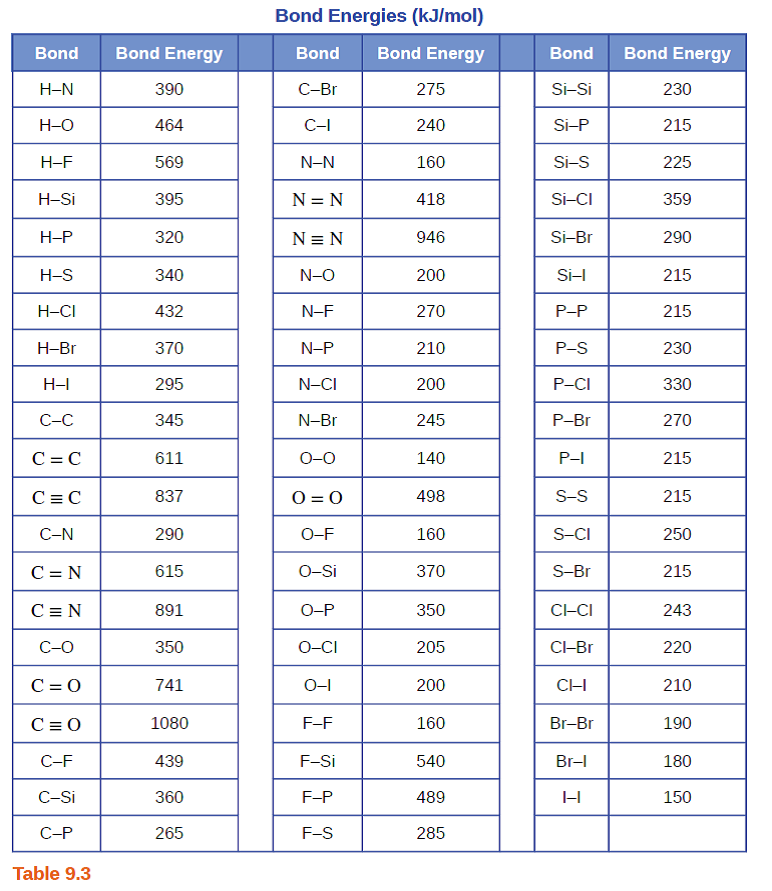
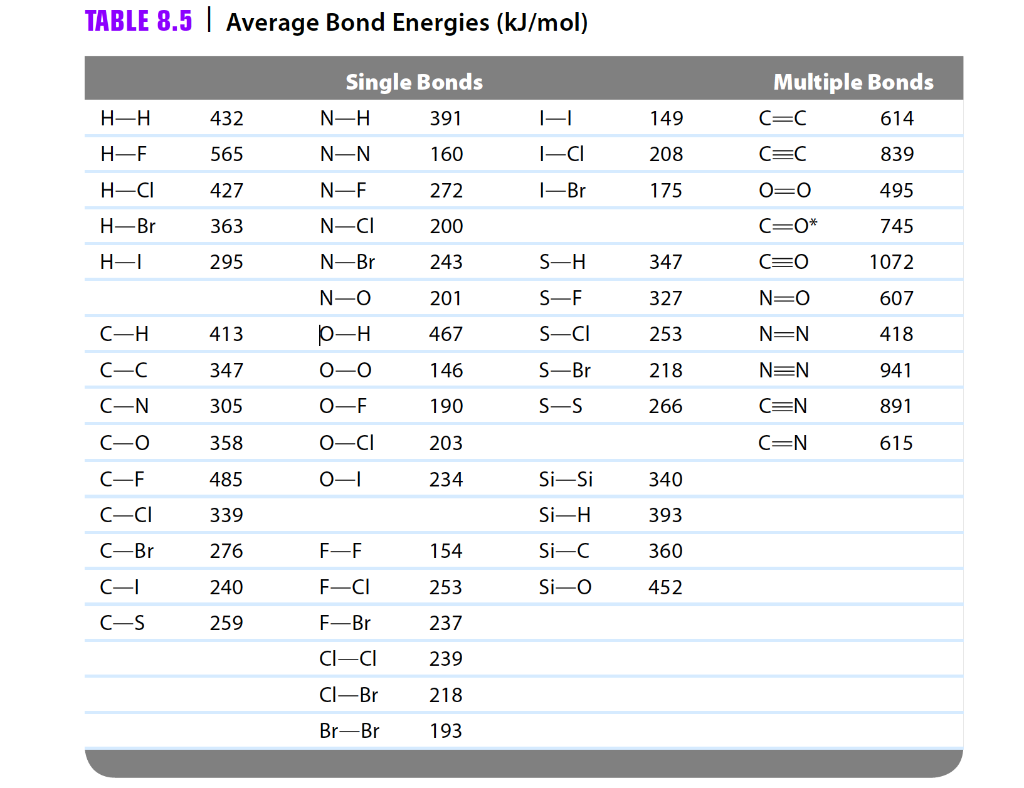
SOLVED: TABLE 9.3 Average Bond Energies Bond Energy (kJ/mol) Bond 436 Bond Energy (kJ/mol) 163 418 946 222 590 272 200 243 159 142 498 190 203 234 159 253 243 Bond Energy (kJ/mol) 237 218 193 208 175 151 323 226 301 265 368 523 464 418 327 253 218 266 ...

Active Thermochemical Tables: Sequential Bond Dissociation Enthalpies of Methane, Ethane, and Methanol and the Related Thermochemistry | The Journal of Physical Chemistry A